You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Coronavirus 2019-2020 thread (no unsubstantiated rumours!)
- Thread starter localizer
- Start date
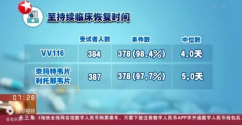
In the trial of VV116, more than 380 people took the experimental drug, while a similarly sized group took Paxlovid. Both treatment courses lasted five days.
The median time to recovery — defined as no Covid symptoms for two consecutive days — was four days for VV116 recipients and five days for those who took Paxlovid. After four weeks, around 98% of all participants had recovered, and no one developed severe Covid.
Study co-author Ren Zhao, a professor at Shanghai Jiao Tong University School of Medicine, called the trial a “great success” in Thursday.
When it comes to specific side effects, around 26% of the trial participants who took Paxlovid said it altered their sense of taste — food tasted sour, sweet, bitter or metallic — but just 4% of people who took VV116 reported that experience. Although some people in both groups had elevated levels of triglycerides (fat in the blood that can increase the risk of heart disease or stroke), a smaller share of those in the VV116 group saw that effect: 11% compared to 21% of participants who took Paxlovid.
The median time to recovery — defined as no Covid symptoms for two consecutive days — was four days for VV116 recipients and five days for those who took Paxlovid. After four weeks, around 98% of all participants had recovered, and no one developed severe Covid.
Study co-author Ren Zhao, a professor at Shanghai Jiao Tong University School of Medicine, called the trial a “great success” in Thursday.
When it comes to specific side effects, around 26% of the trial participants who took Paxlovid said it altered their sense of taste — food tasted sour, sweet, bitter or metallic — but just 4% of people who took VV116 reported that experience. Although some people in both groups had elevated levels of triglycerides (fat in the blood that can increase the risk of heart disease or stroke), a smaller share of those in the VV116 group saw that effect: 11% compared to 21% of participants who took Paxlovid.

Covid-19 situation in the streets of Shanghai
I am going out for a dinner now, let me take a video later. Shanghai had been normal for a week plus man.Covid-19 situation in the streets of Shanghai
The new XBB.1.5 variant is now blowing up in the US and the travel restrictions against China are intended to blame China for it.
This seems like an epic self own. If they didn't have any travel restrictions, they could obfuscate the origin of XBB1.5.The new XBB.1.5 variant is now blowing up in the US and the travel restrictions against China are intended to blame China for it.
But now, it is impossible for them to do it. At most they can say that their travel restrictions were useless, but that makes their own authorities look weak.
Please enlighten us here with your scientific knowledge covid-19 experts @Petrolicious88 @IcmerThe new XBB.1.5 variant is now blowing up in the US and the travel restrictions against China are intended to blame China for it.
Right, so in-vitro experiments indicate the XBB.1.5 variant is significantly more resistant to antibody neutralisation than its BA.2 predecessor. Especially pertinent to immunity and vaccination is that antigenic shift from the XBB.1 sublineages to the original Omicron are comparable to the that of Omicron's from its predecessors. The current vaccines in use are predicted to fair poorly against it, and that immunity from breakthrough infections may be better against infection than purely vaccine based immunity.Please enlighten us here with your scientific knowledge covid-19 experts @Petrolicious88 @Icmer
Link to the study I used.
Quickie
Colonel
Right, so in-vitro experiments indicate the XBB.1.5 variant is significantly more resistant to antibody neutralisation than its BA.2 predecessor. Especially pertinent to immunity and vaccination is that antigenic shift from the XBB.1 sublineages to the original Omicron are comparable to the that of Omicron's from its predecessors. The current vaccines in use are predicted to fair poorly against it, and that immunity from breakthrough infections may be better against infection than purely vaccine based immunity.
Link to the study I used.
And yet South Korea, Italy, and other countries are testing only travelers from China where the XBB.1.5 could be nonexistent or in a tiny percentage.
