I think he meant it breaks the purpose of the sanctionsI'm not sure how these break sanctions either. Even if it does, China isn't going to stop its research, no matter how much natsec types whine. Though Dylan Patel does occasionally have a few insightful comments, he misses a lot more often than not.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chinese semiconductor industry
- Thread starter Hendrik_2000
- Start date
- Status
- Not open for further replies.
For comparison, here are the current top Geekbench 6 and results.
Probably related.Research on wafer stage overlay-μDBO(micro Diffraction Based Overlay) targets by lithography imaging simulation software
1. Front-end System and Integration Engineering Department, Shanghai Micro Electronics Equipment Group Co., Ltd.,(SMEE) Shanghai 201203, China;
2. Metrology Equipment Department, Shanghai Micro Electronics Equipment Group Co., Ltd., (SMEE) Shanghai 201203, China;
3. Shanghai Key Laboratory of Lithographic Optics and Inspection, Shanghai 201203, China
Abstract:Overlay targets with a small range and requiring extremely high measurement accuracy are used in the lithography system's alignment, exposure, and measurement processes. They are inevitably affected by many errors when performing specialized functions, e.g., line width and design difference, line edge roughness, and target edge effect. This paper conducts a simulation study on the micro diffraction-based overlay target (μDBO) in the condition of wafer stage overlay (WSO), which is commonly used in lithography, based on the design of overlay alignment targets in the field of measurement. Litho-target design simulation software is known to be more advanced than lithographic imaging simulation software, but its license is usually rarer and more expensive. We propose a novel method for simulating lithography target metrology results using lithography imaging simulation software. The method predicts the detected light intensity distribution in the experiment qualitatively and can calculate target performance in metrology.
View attachment 121849View attachment 121851
View attachment 121852

two weeks old news but looks like we missed it ..
The carbon material family has added two new members: by performing "anesthesia" and "surgery" on two molecules, Professor Xu Wei's team from the School of Materials Science and Engineering of Tongji University successfully synthesized for the first time a pair of carbon atoms composed of 10 or 14 carbon atoms respectively. Ring-shaped pure carbon molecular material.
According to news from the WeChat public account "School of Materials Science and Technology of Tongji University" on November 30, at 0:00 on November 30, 2023, Beijing time, the top international academic journal "Nature" published online the latest research by Professor Xu Wei's team from the School of Materials Science and Technology of Tongji University. Scientific research results, thesis title is:
This research successfully and accurately synthesized two new carbon molecular materials (carbon allotropes) for the first time, namely aromatic cyclic carbons C10 and C14, and carefully characterized their chemical structures. These two synthesized novel carbon structures It is expected to be used in future molecular electronic devices.
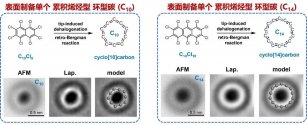
Carbon is a common non-metallic element. Carbon materials come in many forms in nature, and their specific external manifestations depend on the number of atoms bonded to each carbon atom around it. When each carbon atom bonds with four surrounding atoms, it forms a hard and transparent substance that occurs naturally in nature - diamond; when it bonds with three surrounding atoms, it forms soft black graphite.
When each carbon atom bonds to only two surrounding atoms, a ring-shaped pure carbon molecule (ie, cyclic carbon, Cn) is formed. Because this type of carbon structure is highly reactive and extremely unstable, it does not occur naturally in nature and is extremely challenging to synthesize artificially. In addition, the bonding mode between each carbon atom and the two surrounding atoms in cyclic carbons has always been controversial, that is, the cumulative alkene type with equal bond lengths (continuous double bonds) or the polyyne type with unequal bond lengths (single bonds and three-key alternation). Therefore, the most fundamental questions of their puzzling structure and stability have attracted great interest from experimentalists and theorists. Many teams have tried to synthesize cyclic carbons but have not been successful. Although some gas phase experiments have shown signs of the existence of cyclic carbons, it is difficult to isolate, purify and further characterize their structures.
Until 2019, the IBM laboratory and the Oxford University research team prepared a single cyclic carbon C18, and for the first time experimentally verified that C18 has a polyacetylene structure with alternating single and triple bonds. However, cyclic carbons are a large family, and for smaller cyclic carbons, their synthesis becomes more challenging due to their small size. Furthermore, their structure and stability remain elusive. Specially, some theories predict that C10 is the dividing point between cyclic (n ≥ 10) and linear (n < 10), and is also the largest aromatic cumulative olefinic cyclic carbon. C14 is predicted to be the Peierls phase transition state from accumulated olefin type C10 to polyyne type C18. Therefore, studying the structure and stability of C10 and C14 is of extremely important significance. Only by accurately synthesizing these two new carbon material family members can their structures be characterized in detail.
In this study, the team adopted a synthetic route different from C18, which uses cyclic carbon oxides as precursors. Instead, they innovatively designed two precursor molecules, perhalogenated naphthalene (C10Cl8) and anthracene (C14Cl10). Put these two molecules on the "operating table" (sodium chloride film) and "anesthetize" them (freeze them with liquid helium at 4.7 K), and then use the STM tip as a "scalpel" to "surge" them (atomic manipulation), and then induced the complete dehalogenation of the two molecules and the accompanying retro-Bergman ring-opening reaction, and finally successfully synthesized two aromatic ring carbons, C10 and C14, on the surface. Chemical bond-resolved atomic force microscopy shows that, unlike the previous polyyne-type structure of C18, both C10 and C14 have accumulated olefin-type structures. The team further found through theoretical calculations that these two new members of the carbon material family do not have exactly the same characteristics. C10 has no bond length alternation at all, and C14, as the transition state from the accumulated olefin type C10 to the polyyne type C18, has a very special The small bond length alternation (0.05 Å) has not yet reached the form of single bond and triple bond, and cannot be distinguished experimentally.
Professor Xu Wei said that this research work has greatly promoted the development of the field of cyclic carbons, and the proposed surface synthesis strategy is expected to become a universal method for the synthesis of a series of cyclic carbons. At the same time, the synthesized cyclic carbon is expected to be developed into a new type of semiconductor material and has broad application prospects in molecular electronic devices.
The School of Materials Science and Engineering of Tongji University is the only unit that completed the paper. Professor Xu Wei is the only corresponding author. Team members Dr. Sun Luye and distinguished researcher Zheng Wei are the co-first authors. This work was funded by the National Natural Science Foundation of China’s Outstanding Youth Science Fund.
It is expected to become a new semiconductor material! Chinese scientists synthesize new carbon molecule
The carbon material family has added two new members: by performing "anesthesia" and "surgery" on two molecules, Professor Xu Wei's team from the School of Materials Science and Engineering of Tongji University successfully synthesized for the first time a pair of carbon atoms composed of 10 or 14 carbon atoms respectively. Ring-shaped pure carbon molecular material.
According to news from the WeChat public account "School of Materials Science and Technology of Tongji University" on November 30, at 0:00 on November 30, 2023, Beijing time, the top international academic journal "Nature" published online the latest research by Professor Xu Wei's team from the School of Materials Science and Technology of Tongji University. Scientific research results, thesis title is:
This research successfully and accurately synthesized two new carbon molecular materials (carbon allotropes) for the first time, namely aromatic cyclic carbons C10 and C14, and carefully characterized their chemical structures. These two synthesized novel carbon structures It is expected to be used in future molecular electronic devices.

Carbon is a common non-metallic element. Carbon materials come in many forms in nature, and their specific external manifestations depend on the number of atoms bonded to each carbon atom around it. When each carbon atom bonds with four surrounding atoms, it forms a hard and transparent substance that occurs naturally in nature - diamond; when it bonds with three surrounding atoms, it forms soft black graphite.
When each carbon atom bonds to only two surrounding atoms, a ring-shaped pure carbon molecule (ie, cyclic carbon, Cn) is formed. Because this type of carbon structure is highly reactive and extremely unstable, it does not occur naturally in nature and is extremely challenging to synthesize artificially. In addition, the bonding mode between each carbon atom and the two surrounding atoms in cyclic carbons has always been controversial, that is, the cumulative alkene type with equal bond lengths (continuous double bonds) or the polyyne type with unequal bond lengths (single bonds and three-key alternation). Therefore, the most fundamental questions of their puzzling structure and stability have attracted great interest from experimentalists and theorists. Many teams have tried to synthesize cyclic carbons but have not been successful. Although some gas phase experiments have shown signs of the existence of cyclic carbons, it is difficult to isolate, purify and further characterize their structures.
Until 2019, the IBM laboratory and the Oxford University research team prepared a single cyclic carbon C18, and for the first time experimentally verified that C18 has a polyacetylene structure with alternating single and triple bonds. However, cyclic carbons are a large family, and for smaller cyclic carbons, their synthesis becomes more challenging due to their small size. Furthermore, their structure and stability remain elusive. Specially, some theories predict that C10 is the dividing point between cyclic (n ≥ 10) and linear (n < 10), and is also the largest aromatic cumulative olefinic cyclic carbon. C14 is predicted to be the Peierls phase transition state from accumulated olefin type C10 to polyyne type C18. Therefore, studying the structure and stability of C10 and C14 is of extremely important significance. Only by accurately synthesizing these two new carbon material family members can their structures be characterized in detail.
In this study, the team adopted a synthetic route different from C18, which uses cyclic carbon oxides as precursors. Instead, they innovatively designed two precursor molecules, perhalogenated naphthalene (C10Cl8) and anthracene (C14Cl10). Put these two molecules on the "operating table" (sodium chloride film) and "anesthetize" them (freeze them with liquid helium at 4.7 K), and then use the STM tip as a "scalpel" to "surge" them (atomic manipulation), and then induced the complete dehalogenation of the two molecules and the accompanying retro-Bergman ring-opening reaction, and finally successfully synthesized two aromatic ring carbons, C10 and C14, on the surface. Chemical bond-resolved atomic force microscopy shows that, unlike the previous polyyne-type structure of C18, both C10 and C14 have accumulated olefin-type structures. The team further found through theoretical calculations that these two new members of the carbon material family do not have exactly the same characteristics. C10 has no bond length alternation at all, and C14, as the transition state from the accumulated olefin type C10 to the polyyne type C18, has a very special The small bond length alternation (0.05 Å) has not yet reached the form of single bond and triple bond, and cannot be distinguished experimentally.
Professor Xu Wei said that this research work has greatly promoted the development of the field of cyclic carbons, and the proposed surface synthesis strategy is expected to become a universal method for the synthesis of a series of cyclic carbons. At the same time, the synthesized cyclic carbon is expected to be developed into a new type of semiconductor material and has broad application prospects in molecular electronic devices.
The School of Materials Science and Engineering of Tongji University is the only unit that completed the paper. Professor Xu Wei is the only corresponding author. Team members Dr. Sun Luye and distinguished researcher Zheng Wei are the co-first authors. This work was funded by the National Natural Science Foundation of China’s Outstanding Youth Science Fund.
I think he meant it breaks the purpose of the sanctions
No, Patel meant exactly what he said. CMXT breaks sanctions by researching sub 18nm processes. He thinks (or hopes) the US will explicitly sanction CMXT.
That said, any Chinese company that won't advance itself to avoid US sanctions is dead in the Chinese marketplace anyways. So unless CMXT wants to die, it'll need to break sanctions.
CETC and Beijing University are working in a magnetic alignment system for wafer 3D packaging bonding equipment.
Magnetic alignment technology for wafer bonding
Abstract
Purpose
Wafer bonding is a key process for 3 D advanced packaging of integrated circuits. It requires very high accuracy for the wafer alignment. To solve the problems of large movement stroke, position calibration error and low production efficiency in optical alignment, this paper aims to propose a new wafer magnetic alignment technology (MAT) which is based on tunnel magneto resistance effect. MAT can realize micro distance alignment and reduces the design and manufacturing difficulty of wafer bonding equipment.
Design/methodology/approach
The current methods and existing problems of wafer optical alignment are introduced, and the mechanism and realization process of wafer magnetic alignment are proposed. Micro magnetic column (MMC) marks are designed on the wafer by the semiconductor manufacturing process. The mathematical model of the space magnetic field of the MMC is established, and the magnetic field distribution of the MMC alignment is numerically simulated and visualized. The relationship between the alignment accuracy and the MMC diameter, MMC remanence, MMC thickness and sensor measurement height was studied.
Findings
The simulation analysis shows that the overlapping double MMCs can align the wafer with accuracy within 1 µm and can control the bonding distance within the micrometer range to improve the alignment efficiency.
燕东微使用募集资金投资“基于成套国产装备的特色工艺12英寸集成电路生产线项目”,由全资子公司北京燕东微电子科技有限公司实施,项目总投资75亿元,目标为月产能4万片,工艺节点为65nm,产品定位为高密度功率器件、显示驱动 IC、电源管理 IC、硅光芯片等。
该项目周期的一阶段为2023年4月试生产,2024年7月产品达产;二阶段为2024年4月试生产,2025年7月项目达产。
what's does it means "成套国产装备" ? Does it including scanner? are they using domestic dry scanner for production?
该项目周期的一阶段为2023年4月试生产,2024年7月产品达产;二阶段为2024年4月试生产,2025年7月项目达产。
what's does it means "成套国产装备" ? Does it including scanner? are they using domestic dry scanner for production?
Exclusive: After US curbs, Tencent and small chip designers chase Nvidia's China crown
I don't know how credible is this article, but at least there is a lot of specific info on Chinese AI startups
California-based Nvidia commands as much as 90% of China's $7 billion market for chips used to process enormous amounts of data to develop artificial intelligence (AI) software.
However, U.S. strategic technology controls that have emboldened even smaller names such as state-backed Hygon Information Technology and startup Iluvatar CoreX to take the fight to the U.S. goliath.
One of the people said Tencent is pitching its Zixiao v1 variant as a cheaper substitute for Nvidia's A10, used for image and speech recognition AI applications. It is also pushing an upcoming v2Pro variant optimised for AI training as capable of replacing Nvidia's now-blocked L40S, the person said.
Tencent-backed Enflame, which has an AI training accelerator chip called Yunsui, and Iluvatar CoreX, which makes the Tiangai graphics processing unit (GPU), have also been promoting upcoming upgrades of their offerings as substitutes for Nvidia's advanced A100 chip, two of the people said.
"The (United States') original goal was to slow down China's AI capabilities but, in fact, related action has boosted China's self-development capability," he said.
燕东微使用募集资金投资“基于成套国产装备的特色工艺12英寸集成电路生产线项目”,由全资子公司北京燕东微电子科技有限公司实施,项目总投资75亿元,目标为月产能4万片,工艺节点为65nm,产品定位为高密度功率器件、显示驱动 IC、电源管理 IC、硅光芯片等。
该项目周期的一阶段为2023年4月试生产,2024年7月产品达产;二阶段为2024年4月试生产,2025年7月项目达产。
what's does it means "成套国产装备" ? Does it including scanner? are they using domestic dry scanner for production?
If its completely domestic, could be, I know the Shanghai Integrated Circuit Equipment Materials Industry Innovation Center is running purely domestic fabs with SMEE scanners, Naura and others equipment manufacturers tools but these are small-medium production runs to test equipments, materials, parts and solve production problems.
- Status
- Not open for further replies.
