China's new coronavirus cases drop, world still scared
Community workers distribute free vegetables to members of households inside the residential compound, following the outbreak of the novel coronavirus in Wuhan
By Ryan Woo and John Geddie
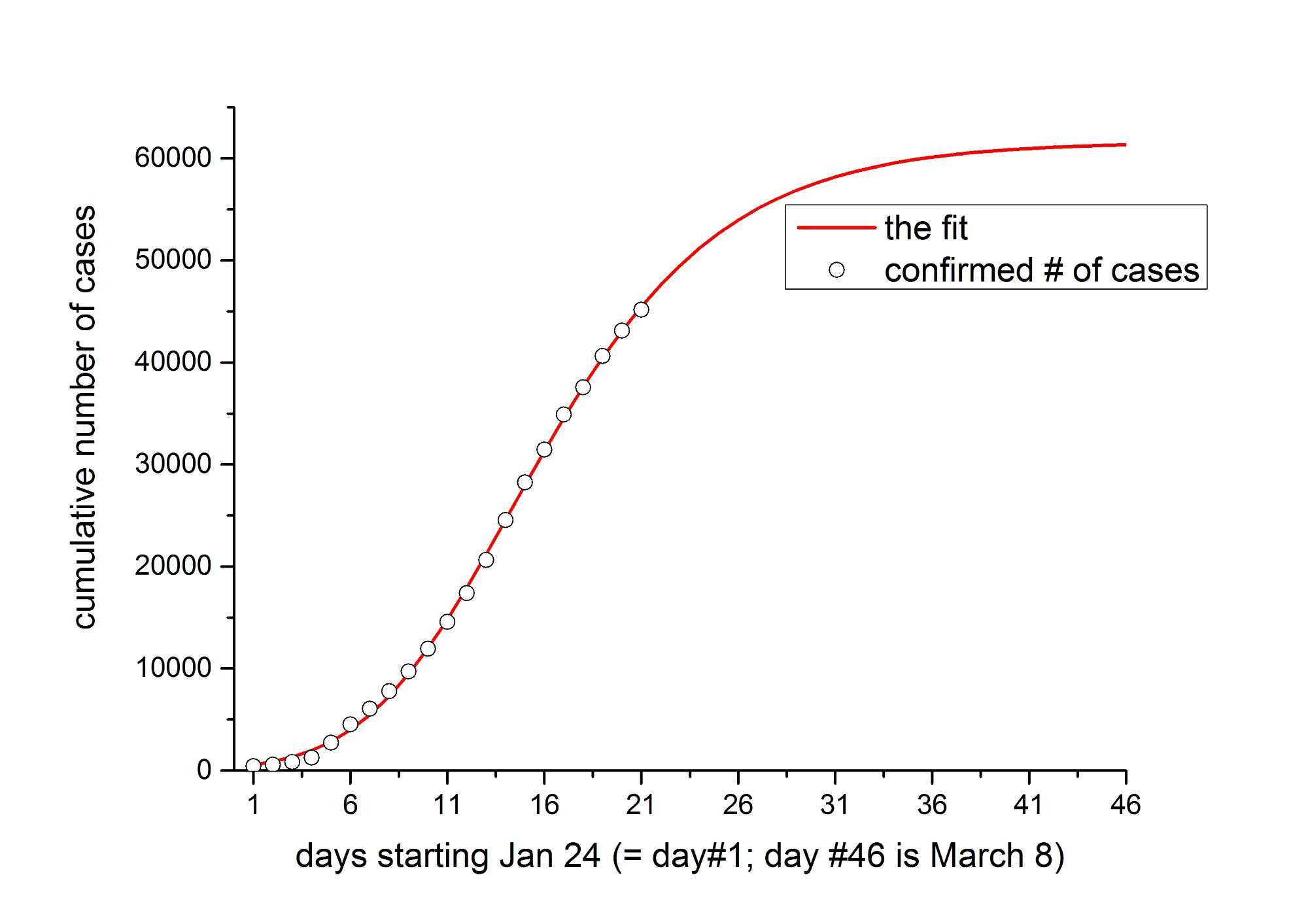
BEIJING/SINGAPORE (Reuters) - China reported on Wednesday its lowest number of new coronavirus cases in two weeks, bolstering a forecast by Beijing's senior medical adviser for the outbreak in the country to end by April - but fears of further international spread remained.
The 2,015 new confirmed cases took China's total to 44,653. That was the lowest daily rise since Jan. 30 and came a day after epidemiologist Zhong Nanshan said the epidemic should peak in China this month before subsiding.
His comments gave some balm to public fears and to markets, where global stocks surged to record highs on hopes of an end to disruption in the world's second largest economy.
But the World Health Organization (WHO) has likened the epidemic's threat to terrorism and one expert said that while it may be peaking in China, this was not the case beyond.
"It has spread to other places where it's the beginning of the outbreak," Dale Fisher, head of the Global Outbreak Alert and Response Network coordinated by the WHO, said in an interview in Singapore. "In Singapore, we are at the beginning."
Singapore has 50 cases, including one found at its biggest bank, DBS <DBSM.SI>, on Wednesday that caused an evacuation at head office.
Hundreds of infections have been reported in dozens of other countries and territories, but only two people have died outside mainland China: one in Hong Kong and another in the Philippines.
China's latest figures also showed that the number of deaths on the mainland rose by 97 to 1,113 by the end of Tuesday.
But doubts have been aired on social media about how reliable the data is, after the government last week amended guidelines on classification.
A Chinese Drugmaker Has Started Mass-Producing an Experimental Drug for COVID-19
Bloomberg
February 12, 2020, 5:35 AM UTC
A Chinese drugmaker said it has started mass-producing an experimental drug from Gilead Sciences that has the potential to fight the novel coronavirus, as China accelerates its effort to find a treatment for the widening outbreak.
Suzhou-based BrightGene Bio-Medical Technology said in a statement filed to the Shanghai Stock Exchange on Tuesday night that it has developed the technology to synthesize the active pharmaceutical ingredients of remdesivir, Gilead’s drug that is a leading candidate to treat the highly-infectious virus that’s killed more than 1,000 people. The drug isn’t licensed or approved anywhere in the world yet.
Its stock surged 20% in Tuesday morning trading in Shanghai.
While BrightGene said that it intends to license the drug from Gilead, its move to start manufacturing at this early stage is highly unusual and a potential infringement of the American company’s intellectual property. It comes a week after Chinese researchers filed an application to patent the drug to treat the new coronavirus, a bid that would give China sway over the global use of the therapy to fight the outbreak.
Large areas of China have been paralyzed by the coronavirus epidemic, and Gilead’s drug is seen as a potential breakthrough after it showed signs of working on infected patients in the U.S. Chinese researchers are now testing the drug on 761 patients in clinical trials in Wuhan.
BrightGene said it will have to license the patent from Gilead, conduct clinical trials and procure regulatory approvals before it can sell the drug on the market. The technology it developed to make remdesivir may not be of much value if the drug fails to produce ideal results from the ongoing clinical trials, or if the epidemic comes under control soon, it said.
Gilead did not immediately respond to a request for comment on BrightGene’s announcement. Last week, the company said it invented remdesivir and has patented it in China, including filing patent applications for use on coronaviruses. The company also said that it is working with Chinese, U.S. and World Health Organization officials to rapidly determine whether the drug can be used to treat the virus.
BrightGene did not immediately respond to Bloomberg queries on its remdesivir production.