Synthesis of TNTNB represents new energy peak for organic explosives
by Bob Yirka , Phys.org
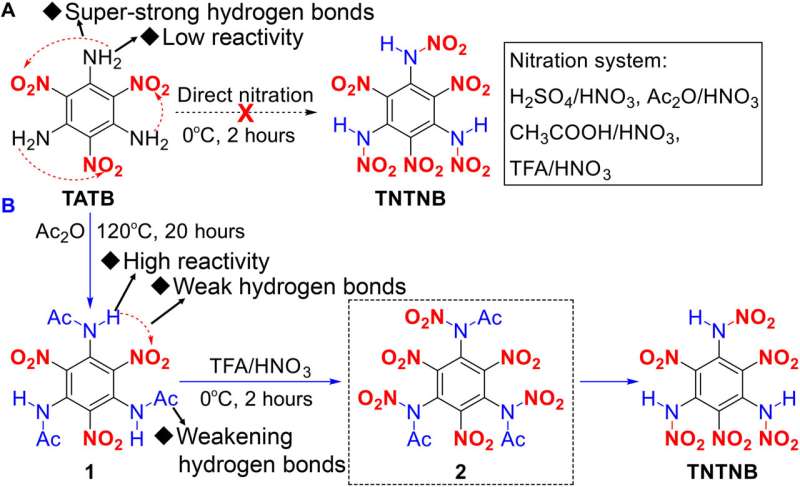
Synthesis of TNTNB.(A) Unsuccessful nitration from TATB to TNTNB using various nitration systems. (B) Synthesis of TNTNB using the proposed acylation-activation-nitration strategy. Credit:
Science Advances (2022). DOI: 10.1126/sciadv.abn3176
A team of researchers from the Beijing Institute of Technology and the China Academy of Engineering Physics has developed a way to synthesize 1,3,5-trinitro-2,4,6-trinitroaminobenzene (TNTNB), marking a new energy peak for organic explosives. In their paper published in the journal
Science Advances, the group describes how they synthesized the powerful explosive and outline its heat detonation levels.
TNT is a well-known and highly
formed from a toluene by substituting its nitro rings with three
. In this new effort, the researchers in China developed an even more powerful organic explosive by combining an oxidizer and a
into a molecular entity. Such an entity, the researchers note, can generate an enormous amount of energy in a short period of time as part of a self-redox reaction. They note that like HNB, another highly explosive compound, TNTNB, has six nitro groups—but it also has three high-energy N-N bonds. Testing has shown that TNTNB produces and releases more heat during detonation than TNT and most other explosives. Its reaction speed has also been measured at 9510 m/s, which makes its detonation velocity faster than HNB. The researchers also note that its sensitivity to friction, shock or electrostatic charge is in the same range as diazodinitrophenol and lead azide, and that it remains stable when exposed to acid, water and air bases due to its three nitroamino groups.
The researchers synthesized the new compound from trinitro-triaminobenzene, which was first created as far back as 1888. Since that time, it has been considered a synthetic dead end for use as an explosive because it has such strong hydrogen bonds that cannot be substituted for nitro groups. With this new effort, the researchers overcame this problem by using acylation to activate the amino groups—that allowed them to attach the nitro groups. More specifically, the team used an acylation-activation-nitration method to synthesize the new compound. They suggest that like other similar explosives, TNTNB could play an important role in mining and engineering applications, pyrotechnics,
and of course in a wide variety of military weapons systems.