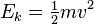optionsss
Junior Member
Well, I think you can just use force divided by the cross-section of the barrel to calculate the pressure. (This is by assuming that the pressure is the same in all direction everywhere in the barrel.)
I think a better way to calculate the pressure is to use the adiabatic expansion (no heat exchange between the gas in the barrel and the surrounding) of the ideal gas. Then we know that Pressure*(Volumn^gamma)=constant, which gives us a relation between pressure and volume. Then integrating Pressure*dVolume from the start to the end of the barrel will give us the kinetic energy obtained by the round. This will help fixing the initial pressure in the barrel. (Roughly speaking, as the volume of the gas increases, the pressure decreases in the barrel as the round moves forward, the temperature of the gas decreases, and the gas looses internal energy, which is converted to the kinetic energy obtained by the round.)
This is as far as I can go. If someone here knows the correct initial condition (pressure, volume or temperature) then he/she can continue further…
Yeah, I know I did a very crude est about the pressue, If there is any really good programmer here, he/she can write a simple program to simulate the whole process with better data, a seasoned programmer can do this is about an hour and have a much accurate result. What I did was just pointing out the fact, moving small object faster and over a longer distance will add that much strain to the barrel than moving a heavier object slower and over a shorter distance, as long as their KE is about the same and no NRG was lost(imposible, i know). And the number are just there to make it more clear.
Last edited: